Utilization of Focused Ultrasound for Reversible opening and Disruption of the Blood-Nerve-Barrier
Daniel Umansky, MD1, Chenchen Bing, PhD1, Tak Ho Chu, PhD1, Samuel Pichardo, PhD1 and Rajiv Midha, MD, MSc2, (1)University of Calgary, Calgary, AB, Canada, (2)Department of Clinical Neurosciences, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
Objective:
In the PNS, endoneurial microvessels directly contact the circulating blood forming the blood-nerve-barrier (BNB). Focused Ultra Sound (FUS) utilization to disrupt the BNB has not been investigated. Circulating microbubbles (MBs) have been shown to amplify FUS cavitation process by acoustic-actuated harmonic extraction and expansion thus providing physical stimulation to the endothelial cells followed by temporary tight junction disruption. This study aims to evaluate the feasibility of using FUS and MBs to achieve BNB disruption in a rodent model for localized delivery of therapeutic agents into the PNS.
Methods:
Adult female transgenic Thy1-GFP SD rats were used in the current study, where the sciatic nerve was surgically exposed for precise target localization. Evans blue dye was injected 5 minutes before a bolus injection of MB (20-40 µl/kg, Definity, Lantheus). Pulsed FUS exposures were delivered immediately after the injection using a benchtop FUS system (RK50, FUS Instruments) at 476.5 kHz. The pulse width was 10ms with 1 Hz repetition frequency over 120 sec. The peak pressure ranged from 0.3 - 0.5 MPa (n = 3/1 for 0.3/0.5MPa) to investigate the effective pressure threshold. Acoustic emissions during the exposures were collected via a passive cavitation detector. Nerves were harvested after treatment and histology was performed to evaluate the treatment effect.
Results:
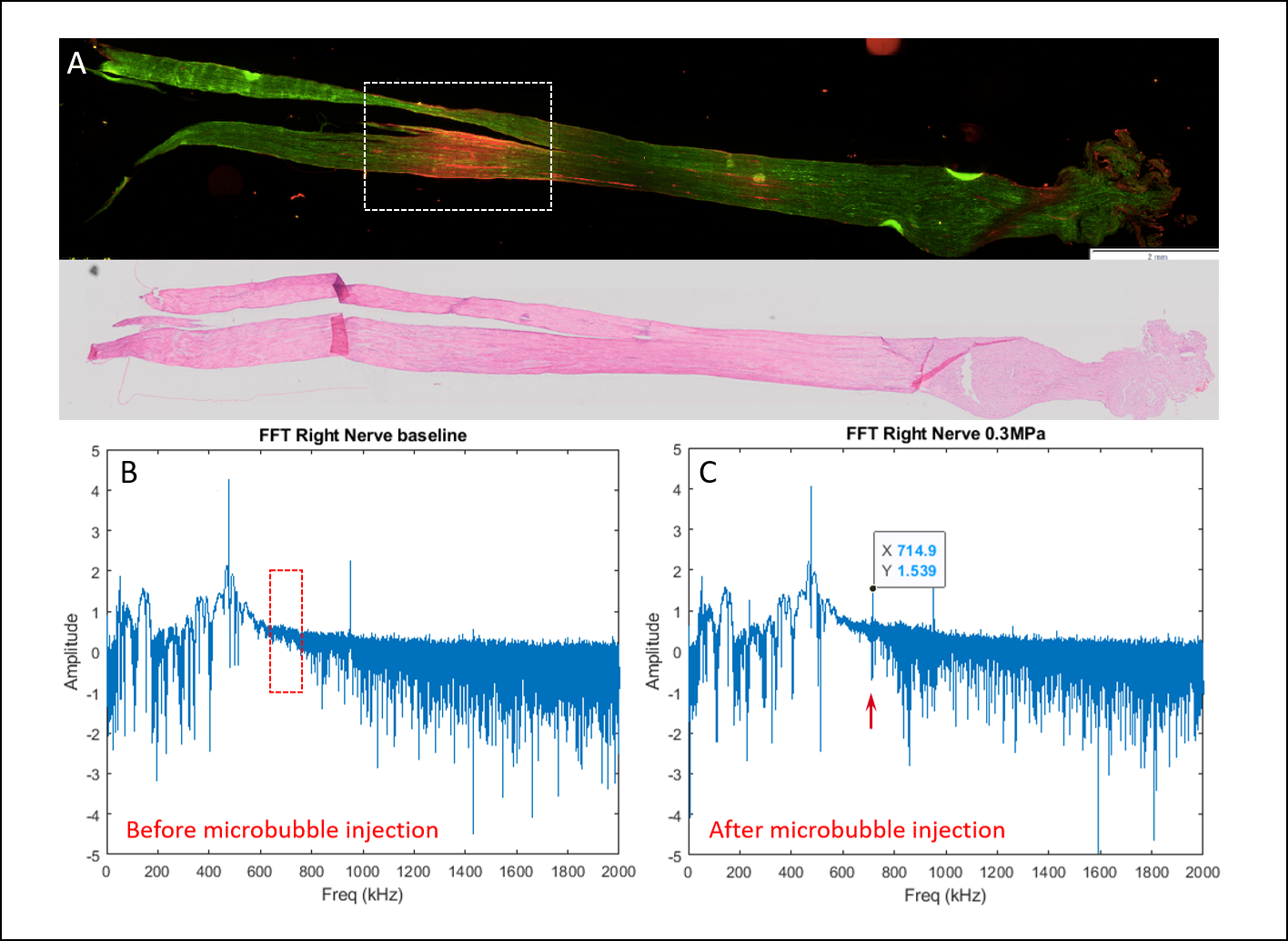
Evans Blue leakage was confirmed at the target region in histological fluorescence evaluation (Figure 1A). Following MB injection, ultra-harmonic signals were detected during FUS exposures, indicating effective bubble responses (Figure 1C) in comparison to baseline reference (Figure 1B) of passive cavitation detector.
Conclusion:
Disruption of BNB in the PNS was achieved using focused ultrasound and microbubbles in a rodent model. A slightly higher dosage (40 µl/kg) might be required to achieve reliable BNB disruption compared to traditional blood-brain barrier disruption treatment protocols.
Ongoing work encompassing optimal threshold and concentrations will be presented in the meeting
Figure 1. BNB disruption effect and acoustic emissions during FUS exposures. A- Fluorescence microscopy scan of targeted nerve, dotted section corresponds to acquired target. Red fluorescence confirms EB leak and BNB disruption (Upper panel). H&E section further confirms BNB disruption without evidence of blood vessel injury or hemorrhage. B- Passive cavitation detection- FUS harmonics baseline reference prior to MB injection. C- Passive cavitation detection- FUS harmonics following MB injection demonstrating ultra-harmonic detection.
Back to 2021 ePosters