Defined Extracellular Matrix Based Microporous Matrices Can Modulate Inflammation, Angiogenesis and Repair in Ex-Vivo Rat Dorsal Root Ganglia
Alan J Hibbitts, PhD1, Zuzana Koci, PhD2, Simone Kneafsey, BSc2, Amos Matsiko, PhD2, Brenton Cavanagh, PhD2, Simon J Archibald, PhD3 and Fergal J O'Brien, PhD2, (1)Royal College of Surgeons Ireland, Dublin, Ireland, (2)Royal College of Surgeons in Ireland, Dublin, Ireland, (3)Integra LifeSciences, Plainsboro, NJ
Introduction:
Biomaterial nerve guidance conduits are an attractive means to repair peripheral nerve injuries. However, healing can be limited due to substrates lacking the necessary pro-repair signalling cues found within the native nerve extracellular matrix (ECM). Here, we investigate the possibility of altering the inflammatory, angiogenic and neurogenic response of regenerating peripheral nerves by altering combinations of the ECM macromolecules. Specifically, we investigate the utility of several fibronectin, laminin 1 and laminin 2 (referred to as “FibL1L2”) combinations when incorporated into a 3D collagen-chondroitin sulphate (Coll-CS) microporous matrix.
Materials & Methods:
Coll-CS mixes were blended with/without varying concentrations of fibronectin, laminin 1 and laminin 2, snap cooled and freeze dried to produce aligned, microporous matrices. Matrices were characterised for alignment and porosity using scanning electron microscopy. Effects of ECM combinations on neuro-inflammation, angiogenesis and repair were assessed at the gene and protein level using adult primary rat dorsal root ganglia (DRG) cultures over 14 days.
Results:
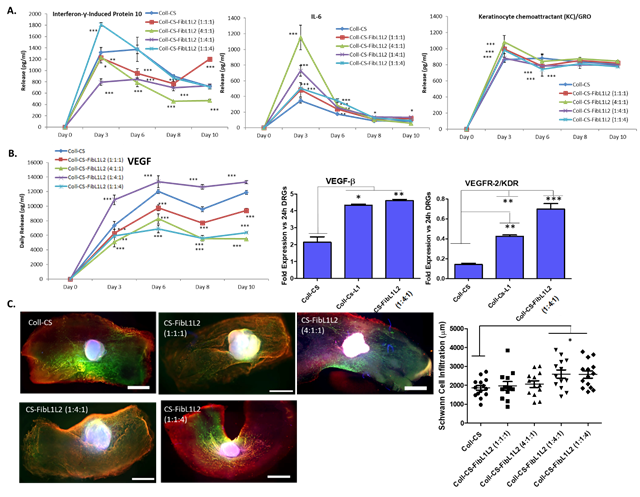
Overall it was found that small alterations in the ECM composition of matrices resulted in profound alterations in behaviour. Inflammation was found to be lowest in matrices that contained high amounts of laminin 1 (i.e. Fib:L1:L2 of 1:4:1) (Figure 1A). These matrices also presented the highest levels of pro-angiogenic VEGF secretion. When compared to samples which only contained laminin 1, it was found that the FibL1L2 combination of 1:4:1 remained significantly higher for VEGF-R gene expression Figure 1B). Rates of Schwann cell infiltration were also found to be significantly enhanced in FibL1L2 samples when compared to unmodified Coll-CS samples (Figure 1C).
Figure 1. 14 day culture of rat DRGs on microporous matrices demonstrated significant changes in behaviour depending on the ratios of Fib, L1 and L2 present. This was apparent in A) pro-inflammatory cytokine secretion, B) pro-angiogenic gene and protein expression and C) Schwann cell (green) and neuron (red) cells (nuclei stained blue) infiltration
Conclusions:
Addition of Fibronectin, laminin 1 and laminin 2 combinations significantly altered the repair phenotype of the cultured DRGs. This indicates that is possible to engineer a favourable repair response in nerve guidance conduits. This approach is now undergoing investigation in large defects in pre-clinical models.
Acknowledgements:
Co-funded by Science Foundation Ireland and Integra LifeSciences through the AMBER Research Centre (TP27-1846A1)
Back to 2021 Abstracts