Surgical Angiogenesis Modifies the Cellular Environment of Decellularized Nerve Allografts in a Rat Sciatic Nerve Defect Model
Mana Saffari, MD1,2, Femke Mathot, MD1,2, Allen T. Bishop, MD3, Andre van Wijnen, PhD4 and Alexander Y. Shin, MD5, (1)Radboudumc, Nijmegen, Netherlands, (2)Mayo Clinic, Rochester, MN, (3)Mayo Clinic, Department of Orthopedic Surgery, Rochester, MN, (4)Orthopedic Surgery, Mayo Clinic, Rochester, MN, (5)Department of Orthopedic Surgery, Mayo Clinic, Rochester, MN
Introduction: A well-vascularized bed for nerve grafts may diminish nerve rejection by altering the microenvironment and intricate paracrine mechanisms that control local cellular pathways. The purpose of this study was to determine how surgical angiogenesis alters the cellular environment of decellularized nerve allografts in a rat sciatic nerve defect model.
Materials & Methods: Unilateral sciatic nerve defects of Lewis rats (N=39) were repaired with (i) autografts, (ii) decellularized allografts, or (iii) decellularized allografts wrapped with a superficial inferior epigastric fascial (SIEF) flap to provide vascularization. Animals were evaluated at two weeks (N=5/group) for flow cytometry and gene expression profiles, and at 12 and 16 weeks (N=4/group/time point) for immunohistochemistry. Gene expression was quantified by quantitative polymerase chain reaction (qPCR) analysis of representative biomarkers, including angiogenic, neurotrophic, immunotrophic and extracellular matrix (ECM) genes.
Results: Flow cytometry revealed a significant increase in T helper population (CD4) in SIEF rats, compared to baseline, untreated rats (P=0.02) after one week. Expression of several angiogenic markers, including Cd34, Pecam1/Cd31, Vegfa and Mmp2 (P<0.05 compared to autograft), as well as ECM proteins such as collagen type I (Col1A1) and type III (Col3A1) (P<0.01, compared to allograft) was significantly increased in SIEF samples. The increase in vascularity was also confirmed by immunohistochemistry at 12 and 16 weeks using antibodies against CD34.
Conclusions: Of the 29 collagen types, collagen type I and III, specifically, are believed to provide mechanical support for axonal growth and regeneration after peripheral nerve injury. Surgical angiogenesis of decellularized nerve allografts alters the cellular environment confirmed with immune cells obtained from peripheral blood, gene expression profiles and immunohistochemical staining. These data support our hypothesis that an adipofascial flap plays a role in suppressing nerve fibrosis after injury, by providing both an immune tolerant paracrine environment and increasing angiogenesis to the nerve allograft.
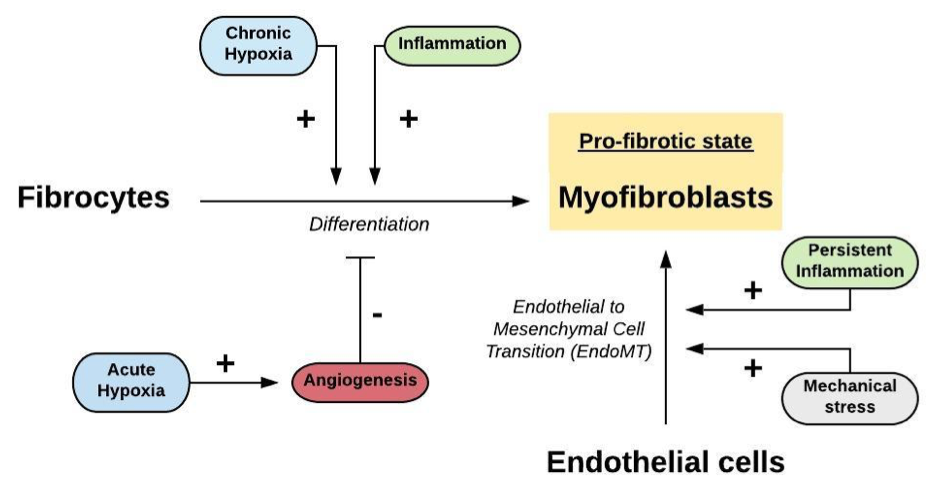
Figure 1. Hypothetical mechanisms that play a role in the conversion of fibrocytes and endothelial cells into myofibroblasts, leading to fibrosis.
Back to 2021 Abstracts