The Adaptive Immune System Has a Critical Role in Promoting Nerve Regeneration across Acellular Nerve Allografts
Matthew D Wood, PhD1, Deng Pan, BS1, Daniel A Hunter, NS2, Lauren Schellhardt, BA3, Anja Fuchs, Ph.D.2, Alexandra E Halevi, MD1, Alison K Snyder-Warwick, MD4 and Susan E Mackinnon, MD5, (1)Washington University School of Medicine, St Louis, MO, (2)Washington University in St. Louis, St Louis, MO, (3)Division of Plastic and Reconstructive Surgery, Washington University in St. Louis School of Medicine, Saint Louis, MO, (4)Plastic Surgery, Washington University School of Medicine, St. Louis, MO, (5)Department of Surgery, Division of Plastic and Reconstructive Surgery, Washington University School of Medicine, Saint Louis, MO
Introduction: Due to disadvantages of nerve autografting, alternatives are being increasingly used and desired for nerve defect repair. But, these alternatives have limitations. As we still do not understand factors critical to promoting regeneration across repaired nerve defects that yields robust functional recovery, we determined whether the adaptive immune system has a role in nerve regeneration across nerve graft alternatives.
Materials and Methods: Acellular nerve allografts (ANAs) were generated using a previously published chemical detergent protocol. One (1) cm ANAs were used to repair sciatic nerve gaps in Rag1KO (lacking both T and B cells) or wild-type (WT; control) mice. Nerve regeneration was quantified using histology, immunofluorescence analysis, retrograde tracing, and a grid walk test (functional outcome).
Results: During early stages of regeneration prior to axon growth across ANAs, T cells (CD3+), but not B cells (CD19+), accumulated within ANAs repairing nerve defects in WT mice. In Rag1KO, Schwann cell (S100) quantities and myelin basic protein (MBP) within ANAs was reduced compared to WT (Fig. 1A, B). Rag1KO demonstrated reduced numbers of myelinated axons, both in the ANA as well as at the distal nerve, compared to WT (Fig. 1C). Furthermore, starting from 4 weeks post repair, Rag1KO had a persistent increase in the proportion of foot faults compared to WT, demonstrating reduced functional recovery (Fig. 1D). Finally, we addressed whether adaptive immune deficiency affected neuron survival using retrograde tracing in injured sciatic nerves of Rag1KO and WT mice. Rag1KO vs WT had no differences in neuron counts after the initial nerve injury.
Conclusions: This data suggests T cells promote regeneration across ANAs.
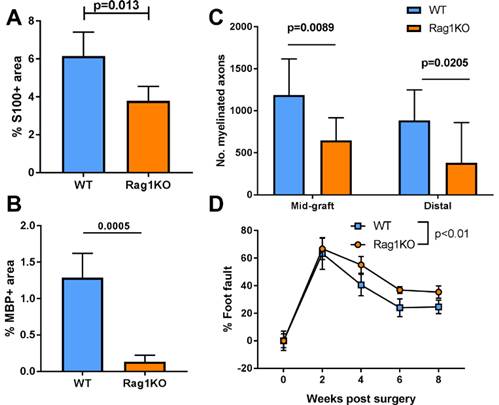 Fig 1 WT vs Rag1KO mice were assessed for nerve regeneration after ANA repair. Mean ± SD, n≥5/group; p values shown.
Fig 1 WT vs Rag1KO mice were assessed for nerve regeneration after ANA repair. Mean ± SD, n≥5/group; p values shown.
Back to 2020 ePosters
