T cells Regulate IL-4, which is Critical to Nerve Regeneration across Acellular Nerve Allografts
Matthew D Wood, PhD1, Deng Pan, BS1, Daniel A Hunter, NS2, Lauren Schellhardt, BA3, Anja Fuchs, Ph.D.2, Alexandra E Halevi, MD1, Alison K Snyder-Warwick, MD4 and Susan E Mackinnon, MD5, (1)Washington University School of Medicine, St Louis, MO, (2)Washington University in St. Louis, St Louis, MO, (3)Division of Plastic and Reconstructive Surgery, Washington University in St. Louis School of Medicine, Saint Louis, MO, (4)Plastic Surgery, Washington University School of Medicine, St. Louis, MO, (5)Department of Surgery, Division of Plastic and Reconstructive Surgery, Washington University School of Medicine, Saint Louis, MO
Introduction: Repair of nerve defects requires the use of a bridging material. There is a desire to transition to alternatives to autografts, such as acellular nerve allografts (ANAs). However, during regeneration in this context, the immune system plays a critical role. Previously, our studies showed that T cells are important for regeneration across ANAs. Now, we address how T cells promote regeneration.
Materials and Methods: ANAs were generated using a chemical detergent protocol. One (1) cm ANAs were used to repair sciatic nerve gaps in Rag1KO, IL-4GFP, IL-4KO, or wild-type (WT; control) mice. As well, in select mice CD4 antibodies were used to deplete CD4 T cells. Regeneration was quantified using qRT-PCR, histology, immunofluorescence analysis, and functional outcome metrics.
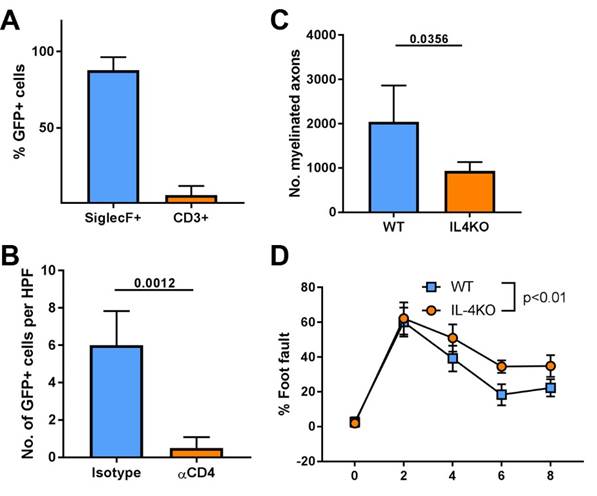
Results: Gene expression analysis of ANAs revealed that Th2 related cytokines, including IL-4, were reduced in Rag1KO vs WT ANAs. The source of endogenous IL-4 was determined using IL-4GFP reporter mice. These mice revealed a correlation between IL-4 expressing cells and T cell (CD3+) accumulation within ANAs. But, eosinophils (Siglec F+), rather than T cells, were the primary source of IL-4 within ANAs (Fig. 1A). Yet, depletion of CD4 T cells reduced accumulation of IL-4 expressing eosinophils in ANAs demonstrating that T cells regulate eosinophils and in turn IL-4 expression within ANAs (Fig. 1B). Finally, nerves repaired using ANAs in IL-4KO had reduced regeneration across the ANA and reduced functional recovery compared to WT establishing the importance of IL-4 signaling to regeneration across ANAs (Fig. 1C,D).
Conclusions: Our data suggest T cells regulate the expression of IL-4 within the ANA to promote regeneration of myelinated axons.
Fig 1 Assessment of the source of IL-4 expression, how it is regulated, and whether IL-4 has an impact on nerve regeneration ANA repair. Mean ± SD, n≥3/group; p values shown.
Back to 2020 Abstracts