Electrophysiological and Histological Assessment of Composite Regenerative Peripheral Nerve Interface Reinnervation
Daniel C Ursu, PhD1, Carrie A Kubiak, MD1, Jana D Moon, BS2, Parag C Patil, MD, PhD1, Theodore A Kung, MD3, Paul S. Cederna, MD4 and Stephen WP Kemp, PhD1, (1)University of Michigan, Ann Arbor, MI, (2)Section of Plastic Surgery, University of Michigan, Ann Arbor, MI, (3)Section of Plastic & Reconstructive Surgery, University of Michigan, Ann Arbor, MI, (4)Department of Surgery, Section of Plastic Surgery, University of Michigan, Ann Arbor, MI
Introduction: There is a fundamental gap in understanding how to provide prosthetic limbs with intuitive afferent somatosensory feedback essential for interaction with the environment, while simultaneously acquiring efferent motor signals for prosthetic control. The composite regenerative peripheral nerve interface (C-RPNI), created by surgically implanting the distal end of a transected peripheral nerve in between an autogenous free muscle and dermal skin graft, is a novel construct designed to overcome this problem. The long-term goal of this research is to develop a single biologic interface where we can record from C-RPNIs to provide high fidelity motor control of a prosthetic limb, while simultaneously stimulating the dermal component of the C-RPNI to provide sensory feedback.
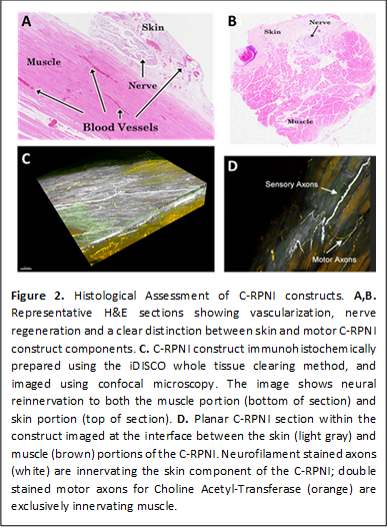
Materials & Methods: C-RPNIs were surgically implanted on the end of the transected peroneal nerves of 16 rats, using free muscle grafts obtained from the animal’s contralateral limb, and de-epithelialized dermal grafts harvested bilaterally from the glabrous skin of 8 donor rat hindpaws. At three and six months post-surgery, the C-RPNI constructs were electrophysiologically evaluated ex-vivo by stimulating: 1) nerve, 2) skin, and 3) muscle, while simultaneously recording signals from: a) muscle and skin, b) nerve and muscle, and c) nerve and skin, respectively. After six months, eight C-RPNI constructs were harvested, and labeled with fluorescent antibodies targeting nerve fibers, neuromuscular junctions, and motor axons using the iDISCO tissue clearing method.
Results: Electrophysiological evaluations at 3 and 6 months revealed muscle, skin and nerve capable of generating peak CMAP amplitudes and conduction velocities of 7.9±2.2 mV and 9.8 ±1.3 m/s, and CSNAPs with 133±30 µV peaks at 9.8 ±1.3 m/s velocity [Fig. 1]. End-point histology displayed healthy vascularized muscle maintaining 73±9% original muscle mass, and preferential migration of sensory and motor fibers into the skin and muscle portions of the construct, respectively [Fig. 2].
Conclusions: Mixed sensorimotor nerves demonstrated preferential motor reinnervation of muscle and sensory reinnervation of skin in the C-RPNI. Electrophysiological evaluations of these constructs revealed stable and appropriate afferent and efferent signals over a six month period, confirming the potential of C-RPNIs to provide closed-loop neural control of prosthetic devices.
Back to 2020 Abstracts