Viability and Signaling of the Muscle Cuff Regenerative Peripheral Nerve Interface
Carrie A Kubiak, MD; Daniel Ursu, PhD; Jana D Moon, BS; Theodore A Kung, MD; Paul S Cederna, MD; Stephen WP Kemp, PhD
University of Michigan, Ann Arbor, MI
Introduction: Robotic exoskeletons have emerged as promising adjuncts for the restoration of functional independence for patients with intact peripheral nerves but poor motor control and strength. An ideal exoskeletal device is able to detect the userŐs intention to execute a maneuver and then elicit the prosthesis to assist in that movement. However, current motor-intent detection technology remains inaccurate and overall unsatisfactory. The Muscle Cuff Regenerative Peripheral Nerve Interface (MC-RPNI) is a novel biologic interface that may allow for a more accurate measurement of the userŐs motor intention. The MC-RPNI is surgically constructed from a muscle graft wrapped around an intact (undivided) peripheral nerve. The MC-RPNI becomes reinnervated and amplifies signals from peripheral nerves without needing to cut the nerve itself. The purpose of the present study was to investigate in vivo stability as well as efferent and afferent signal transduction capability of this novel interface.
Materials & Methods: Twenty F344 rats were randomly assigned to one of four experimental groups: (A) 8 mm MC-RPNI with epineurial window; (B) 8 mm MC-RPNI without epineurial window; (C) 13 mm MC-RPNI with epineurial window, and (D) 13 mm MC-RPNI without epineurial window. MC-RPNIs were surgically created by wrapping free skeletal muscle grafts around the intact right common peroneal nerve. At three months, four electrophysiologic tests were performed. The proximal peroneal nerve was stimulated while efferent signals (CMAPs) were measured from (1) the Muscle Cuff-RPNI and (2) the distal target muscle (EDL). The muscle cuff-RPNI was then stimulated while (3) efferent signals (CMAPs) were recorded from the EDL and (4) afferent signals (CSNAPs) were recorded from the proximal peroneal nerve.
Results: Muscle Cuff-RPNI constructs remained viable over the three-month period and demonstrated robust regeneration and revascularization. Results of electrophysiologic testing is presented in the Table. There were no observed differences between rats with an epineurial window and those without, or those with short versus long MC-RPNIs.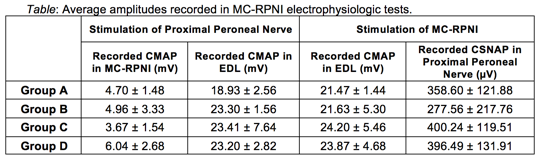
Conclusion: The MC-RPNI is capable of amplifying neuronal signals from intact peripheral nerves to larger, recordable EMG signals. This occurs without adversely impacting the function of the peroneal nerve or the distal muscle. The MC-RPNI also facilitates afferent signal transduction along the proximal nerve. These findings validate the potential of the MC-RPNI to serve as the critical interface to control a robotic exoskeleton.
Back to 2019 Absteracts
