Determining the Potential of Fibrin to Facilitate Schwann Cell Transplant to Acellular Nerve Allografts
Jerry Tsung-Kai Lin, MD1,2; Deng Pan, BS1; Xueping Ee, MD1; Johnny Chuieng - Yi Lu, MD3,4; Matthew Wood, PhD1; Susan E. Mackinnon, MD5
1Washington University School of Medicine, St Louis, MO, 2Department of Surgery, Chang-Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan, 3Washington University in St. Louis, St. Louis, MO, 4Department of Plastic and Reconstructive Surgery, Chang Gung Memorial Hospital, Chang Gung Medical College and Chang Gung University, Taoyuan, Taiwan, 5Department of Surgery, Division of Plastic and Reconstructive Surgery, Washington University School of Medicine, St. Louis, MO
Introduction
Artificial nerve grafts, such as acellular nerve allografts, can be used to repair nerve gaps following peripheral nerve injury. However, the regenerative potential of these grafts is limited by their inherent lack of cells. Supplementing these grafts with cells could further enhance regeneration, but techniques to transplant cells to grafts are underdeveloped. This study developed methods to transplant Schwann cells (SCs) to nerve grafts. Specifically, this study determined whether fibrin could be used to promote transplanted SC survival and facilitate SC transplantation to nerve grafts.
Material and Methods
To evaluate the ability of fibrin gels to effectively transplant cultured SCs, adult Lewis rat sciatic nerves were transected, resulting in a 3mm gap, and the gap repaired using fibrin gels, with and without SCs. These repaired nerves were harvested at 1, 7, and 14 days to evaluate SC survival and nerve regeneration using standard immunohistochemistry techniques. In vitro, acellular nerve grafts were supplemented with fibrin gels and SCs, where transgenic SCs expressing fluorescent reporter were used to quantify SC repopulation of the grafts.
Results
Constructed fibrin gels promoted nerve regeneration across a 3mm gap in rat sciatic nerves. Axons were visualized within the fibrin by 7 days and crossing to the distal nerve by 14 days. Fibrin gels also served as a viable scaffold carrier to facilitate SC transplantation. One day following transplant, SC numbers within fibrin gels were estimated at ~50% of the original transplanted SC numbers. Furthermore, transplanted SCs were evident after 7 days within the fibrin gel and adjacent nerve stumps. These fibrin gels with SCs enhanced nerve regeneration across the short gap, where axons were visualized crossing the gap by 7 days. In vitro, acellular nerve grafts supplemented with fibrin containing SCs were able to achieve SC migration into the graft by 5 days in culture.
Conclusion
Fibrin gels were suitable as a carrier to improve SC survival for transplantation in vivo.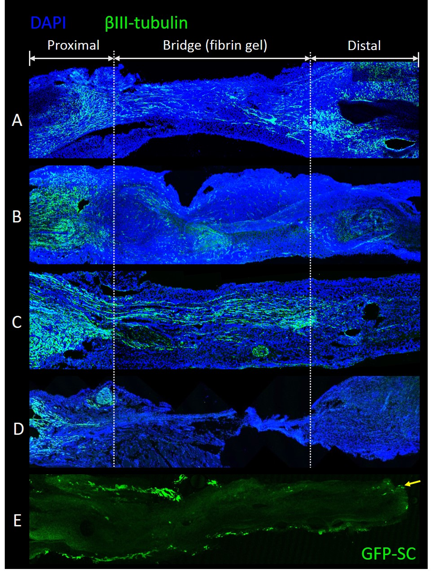
Figure: Axon regeneration of (A) 100,000 Schwann Cells transplanted in fibrin by 7 days (B) Fibrin gel without Schwann Cells by 7 days; (C) Fibrin gel without Schwann Cells by 14 days (D) No fibrin gel by 14 days. (E) Some Schwann cells migrated into the acellular nerve graft from the fibrin gel by 5 days (arrow).
Back to 2019 ePosters
