Functional Characterization of Mixed, Motor, and Sensory Nerve Regenerative Peripheral Nerve Interfaces (RPNIs)
Andrej Nedic, MSE1; Alixandra L VanBelkum, BA2; Nathan G Lawera, Undergraduate1; Vincent Thieu, BSc.1; Zaid Khatib, BSc.1; Melanie G. Urbanchek, PhD2; Paul S Cederna, MD3; Stephen W. P. Kemp, PhD, MSc4; (1)University of Michigan, Ann Arbor, MI, (2)Department of Surgery, Section of Plastic Surgery, University of Michigan, Ann Arbor, MI, (3)Plastic Surgery, University of Michigan, Ann Arbor, MI, (4)Division of Plastic and Reconstructive Surgery, University of Michigan, Ann Arbor, MI
PURPOSE: Approximately 185,000 Americans suffer devastating limb loss yearly, resulting in substantial functional deficits adversely impacting quality of life. Regenerative Peripheral Nerve Interfaces (RPNIs) present a promising surgical strategy for interfacing human volition with myoelectric prostheses. Rat studies led to proof of RPNI long-term function and high signal to noise ratio with no adverse biological effects. However, characterization of different types of nerve RPNIs has not yet been evaluated.
MATERIALS AND METHODS: RPNIs consisted of a small, autologous partial muscle graft reinnervated by a transected peripheral nerve branch. Five experimental groups were analyzed: (1) Motor (femoral nerve, motor branch) RPNI; (2) Sensory (sural nerve) RPNI; (3) Mixed (common peroneal nerve) RPNI; (4) negative control (common peroneal nerve transected and not repaired), and; (5) positive control (surgical na´ve animal). After 3 months of convalescence, terminal EMG measurements were assessed including nerve conduction velocity and compound muscle action potentials (CMAPs). A 5 mm segment proximal to the rpni was harvested and processed for nerve histomorphometry. All RPNI constructs were harvested and processed for Immuno-enabled Three-Dimensional Imaging of Solvent-Cleared Organs (IDISCO) to visualize axonal infiltration into the RPNI. Retrograde labeling was conducted in a separate cohort of animals to determine neuronal numbers innervating individual RPNIs.
RESULTS: At harvest, all RPNIs were well vascularized but smaller in size than when implanted. Nerve conduction velocities and CMAPs were achieved in all three experimental RPNI groups, with mixed nerve and femoral nerve RPNIs displaying greater CMAPs and terminal weights than sensory RPNIs. IDISCO revealed the extensive 3-dimensional patterns of axonal reinnervation of each corresponding RPNI (Fig.1). Retrograde labeling showed differences in neuronal numbers between different RPNI groups.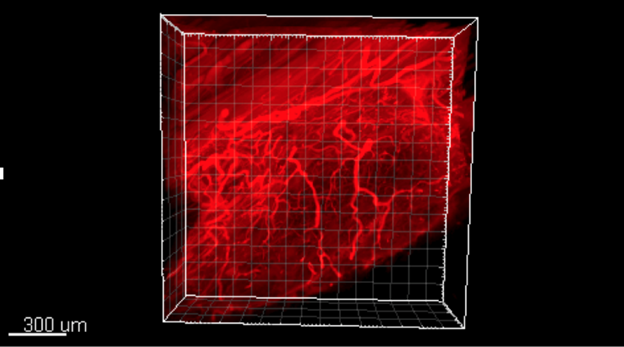
CONCLUSIONS: Motor, sensory, and mixed nerve RPNIs displayed robust neural regeneration. Results from this study will enable us to further evaluate electrode-biological interfaces, and help us develop future prostheses with the ability to respond to both motor and sensory neural signals.
Figure 1. Three-dimensional IDISCO imaging of RPNIs shows robust reinnervation of tissues by nerve of varying size. Fluorescent labeling of neurofilament-M (red) shows that peripheral nerve fibers regenerate diffusely throughout the entire free muscle graft.
Back to 2018 Program
