Back to 2017 Annual Meeting Program
Molecular Basis for the Utilization of a Novel Anti-fibrotic Agent to Minimize Perineural Fibrosis Following Nerve Repair
Andrew Miller, MD1; Tulipan Jacob, MD1; Michael Rivlin, MD2; Pedro Beredjiklian, MD1; Andrzej Fertala, PhD1; Mark Wang, MD1
1Thomas Jefferson University Hospital, Philadelphia, PA, 2Massachusetts General Hospital, Harvard University, Boston, MA
Introduction: Neural scar formation constitutes a major barrier to peripheral nerve regeneration following repair or injury. Extra-neural scarring occurs through myofibroblastic collagen deposition that leads to fibrosis. These adhesions compromise nerve gliding and result in poor function. In contrast, intra-neural scarring results in diversion or blockade of regenerating axons. The goals of this study were to evaluate the pathophysiology of neural fibrosis.
Methodology: We utilized 4 White New Zealand rabbits to create a peripheral nerve injury model. Crush and 50% partial transection injuries were performed to replicate Seddon Grade 2 and 3 nerve injuries. In Phase 1 of the study, we histologically compared the partially transected and crushed nerves two week time intervals and evaluated changes in the healing nerve. We measured the extent of fibrosis by analyzing the expression of Heat Shock Protein (HSP) 47, a collagen-specific chaperone and alpha smooth muscle actin. The percent positivity of HSP47 was calculated in regions of interest.
Results: Fibrotic tissue was heterogeneously concentrated in the epineurium and at the site of injury in the partial transection injury (Figure 1 B). Fibrotic tissue was concentrated homogeneously in the epineurium and endoneurium in the crush injury model (Figure 1 C). The epineurium of both the transection and crush nerve injury models yielded comparable positivity for HSP47 (Graph 1). There was a significant increase in HSP47-positive cells in the crushed nerve injury endoneurium compared to the transection nerve injury endoneurium distant to the site of injury (p< 0.001) (Graph 1). Finally, while aSMA-positive cells were clearly apparent in epineurium of cut and crushed nerves, this marker, except in pericytes, was absent in the endoneurium (Figure 2).
Conclusions: Increased number of HSP47-positive cells suggests that a pro-fibrotic response in peripheral nerves includes activation of a mechanism that controls increased collagen production. The absence of aSMA-positive cells in endoneurium of injured nerves suggests an independent intra-neural fibrotic pathway that may exist outside the myofibroblastic cascade. 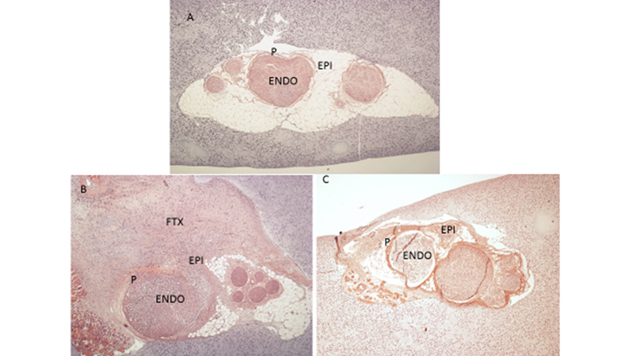
FIGURE 1:
A.) Uninjured nerve on Hematoxylin and Eosin Stain (H&E) B.) 50% Partial Transection Injury at 2 weeks on H&E C.) Crush injury at 2 weeks on H&E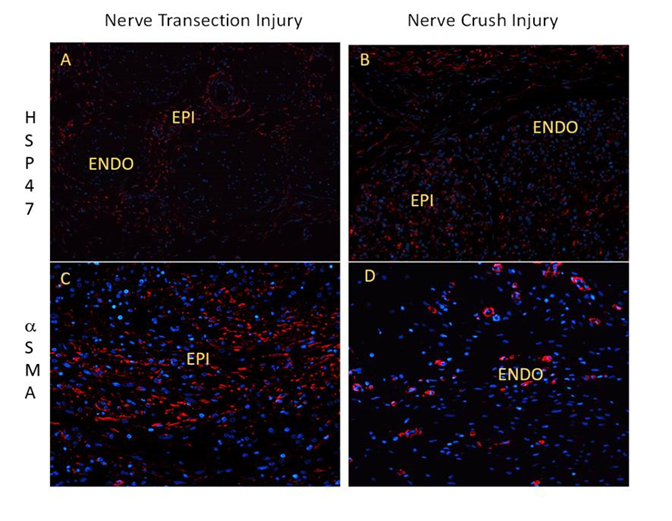
Figure 2:
A.) Transection injury at 2 weeks staining for HSP47 B.) Crush injury at 2 weeks staining for HSP47 C.) Transection Injury at 2 weeks staining for alpha SMA D.) Crush Injury at 2 weeks staining alpha SMA
LEGEND: ENDO (Endoneurium), EPI (Epineurium), P (Perineurium), FTX (Fibrotic Tissue)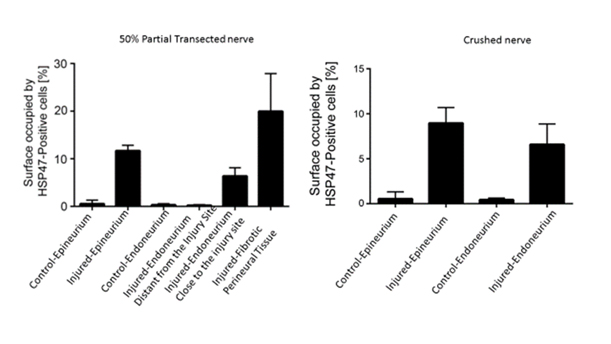
Back to 2017 Annual Meeting Program
