Back to 2017 Annual Meeting Program
Conducting Polymer Nanostructures Facilitate Peripheral Nerve Regeneration
Mohammad Reza Abidian, PhD1; Paul S. Cederna, MD
1University of Houston, Houston, TX , 2University of Michigan, Ann Arbor, MI
Introduction: Autografts are the clinical "gold standard" for bridging peripheral nerve gaps and have been widely used for reconstruction, however, they have some disadvantageous such as painful dysesthesias. Biomaterials have been utilized for design of nerve conduits, but regeneration of functional axons in long nerve gap remains challenging due to the lack of biomaterials that can provide both biochemical and biophysical growth cues. Conducting polymers (CPs) have been considered neural interfaces due to their unique physical, chemical, and electrical properties. We hypothesized that nanostructured CPs can facilitate axonal regeneration by providing guidance cues. To access this hypothesis, we developed a novel method for fabrication of (1) aligned conducting polymer nanotubes (ACPNTs) and (2) hybrid conducting polymer-hydrogel conduits for axonal regeneration.
Materials & Methods: The fabrication process involves the electrospinning of aligned nanofibers into which nerve growth factor (NGF) have been incorporated followed by electrochemical deposition of conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around the nanofibers. Dorsal root ganglion explants, and PC12 cells were cultured on these ACPNTs. Scanning electron and fluorescence microscopy examined the topography and neurite outgrowth. We fabricated hybrid conduit consisting PEDOT and agarose hydrogel. PEDOT was electrodeposited inside the lumen to create a fully coated-PEDOT agarose (FPEDOTA) conduit and a partially coated-PEDOT agarose (PPEDOTA) conduit. These conduits were compared with autograft controls: animals that received plain agarose (PA), FPEDOTA, and PPEDOTA conduits. These conduits were implanted in 10 mm peroneal nerve gaps in rats and compared with autograft after 12 weeks postoperatively. The outcome measures utilized included extensor digitorum longus muscle contractile force measurements, a muscle innervated by the peroneal nerve, and nerve histomorphometry.
Results: In vitro results revealed that neurites were preferentially guided in the direction of the ACPNTs. The Cell culture experiments confirmed retain retained bioactivity of NGF during the fabrication process. We demonstrated that FPEDOTA and PPEDOTA conduits supported superior neural regeneration as compared to the PA conduit. PEDOT polymerization within a hydrogel has been shown to mechanically strengthen the combination.
Conclusions: This study paves the way for the design of a three-dimensional conductive hydrogel scaffold for accelerated, directional, and controlled axonal growth in the peripheral nervous systems. The PEDOT lining may be used to facilitate future studies using electrical stimulation and/or controlled release of neurotrophins. 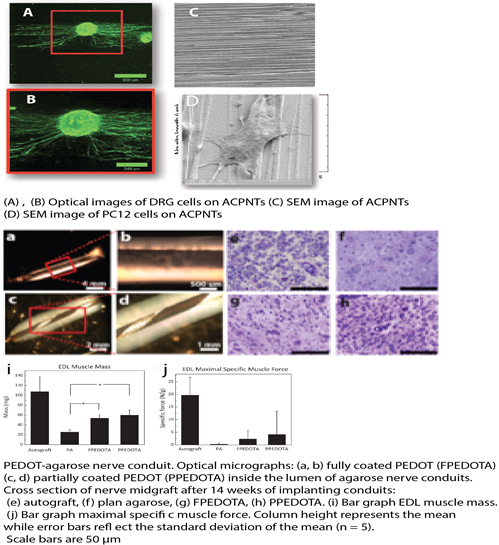
Back to 2017 Annual Meeting Program
