Back to 2015 Annual Meeting Program
Impact of Decellularized Small Intestinal Submucosa on Regeneration, Reinnervation, and Revascularization of Freely Transferred Muscle
Frances M. Walocko, MSE1; Elizabeth A. Mays, BSE1; Cheryl A. Hassett, BS1; Jana D. Moon, BS1; Nicholas B. Langhals, PhD1; Paul S. Cederna, MD1; Melanie G. Urbanchek, PhD2 1Department of Surgery, Section of Plastic Surgery, University of Michigan, Ann Arbor, MI; 2Plastic Surgery, University of Michigan, Ann Arbor, MI
Introduction: To improve reliability of myoelectric prosthetic devices, we developed the Regenerative Peripheral Nerve Interface (RPNI) for neuroprosthetic control. In our standard model, decellularized small intestinal submucosa (SIS) secures an epimysial electrode to the RPNI. However, RPNI viability and signal transduction require muscle regeneration, reinnervation and revascularization, and SIS may affect these essential processes. We determined the effects of SIS on RPNI viability by comparing muscle fiber regeneration, reinnervation, and revascularization of freely transferred muscle wrapped in SIS to those without SIS.
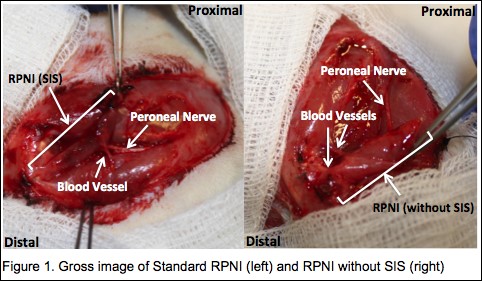
Materials & Methods: Rats were assigned to Standard RPNI (n=6) or RPNI without SIS (n=6) groups. RPNIs were surgically constructed with autologous unilateral, free muscle transfer to the ipsilateral upper thigh using the extensor digitorum longus (EDL) muscle. The proximal end of the divided peroneal nerve was implanted into the muscle. The EDL muscle was then either wrapped in SIS (Standard RPNI) or not (RPNI without SIS) (Figure 1). Three months postoperatively, RPNI compound muscle action potential (CMAP), force, fatigability, and histology were assessed.
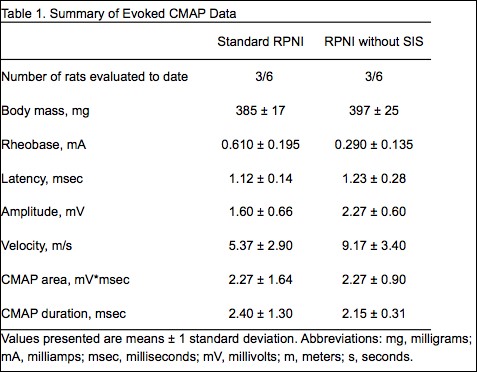
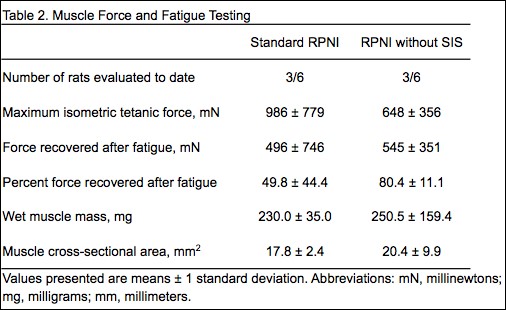
Results: All rats survived the surgery and did not differ in age or body mass. RPNIs without SIS had 42% increased CMAP amplitude and 52% decreased rheobase (minimal current required to reach depolarization threshold), illustrating increased muscle reinnervation (Table 1). Although RPNIs without SIS produced similar maximal forces, surprisingly, they recovered 80% of maximal force after 20-minutes of fatiguing work, while Standard RPNIs only recovered 50% (Table 2). Thus, fatigue testing exposed a previously hidden limitation of the SIS RPNI wrap. The 8.9% greater muscle mass seen in RPNIs without SIS may also indicate enhanced regeneration, reinnervation, and revascularization. Additional RPNI testing, histology, and statistical analyses are in progress.
Conclusion: RPNIs without SIS have improved RPNI muscle viability and signal production during both brief and continuous CMAP activation when compared with Standard RPNIs. With study completion, we shall present complete SIS impact results for regeneration, reinnervation, and revascularization of freely transferred muscle.
Back to 2015 Annual Meeting Program
