Back to 2015 Annual Meeting Program
Effects of Electrode Presence on Regenerative Peripheral Nerve Interface (RPNI) Performance
Zachary P. French; Elizabeth A. Mays, BSE; Frances M. Walocko, BSE, MSE; Cheryl A. Hassett, BS; Jana D. Moon, BS; Nicholas B. Langhals, PhD; Paul S. Cederna, MD; Melanie G. Urbanchek, PhD
Section of Plastic Surgery, University of Michigan, Ann Arbor, MI
Background: A Regenerative Peripheral Nerve Interface (RPNI) is a piece of grafted autologous muscle that regenerates and becomes reinnervated by an implanted residual peripheral nerve. By convention, RPNIs have epimysial electrodes fixed to the muscle by a wrap of decellularized small intestinal submucosa (SIS). RPNIs rival “Targeted Muscle reinnervation” for controlling myoelectric prostheses because RPNI electrodes are in direct contact with muscle instead of skin. Our purpose is to determine whether epimysial electrodes remain stable, record signals, and allow the RPNIs to function optimally by comparison to RPNIs with no electrodes. Methods: In rats (n=13), one extensor digitorum longus (EDL) muscle was transferred to the upper thigh and implanted with the transected peroneal nerve. Two groups were formed by either not placing (NO ELECTRODE) or placing fine wire, pad or patch electrodes (ELECTRODE) on the muscle. A wrap of SIS was placed around each RPNI. Rats recuperated for at least three months. Residual peroneal nerve stimulation was used to evaluate RPNI muscles during needle by compound muscle action potential (CMAP), muscle contractile force, and a 40 minute fatiguing protocol.
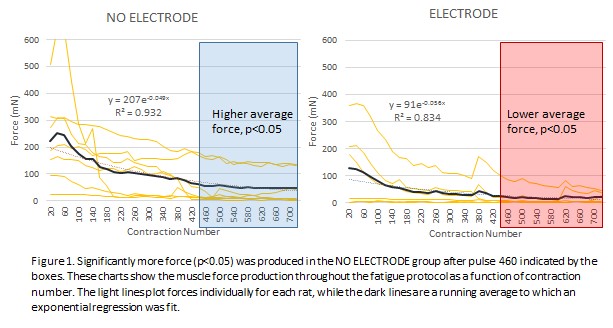
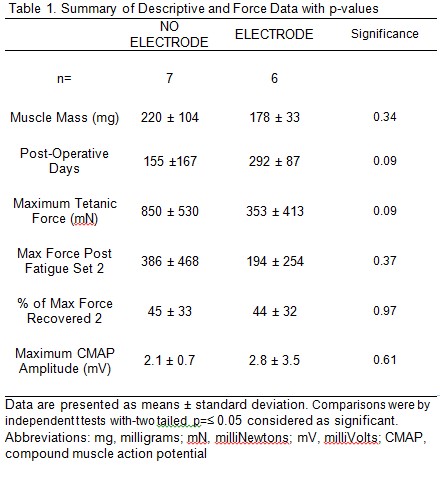
Results: At evaluation, all RPNI muscles of both NO ELETRODE and ELECTRODE groups had a thin connective tissue layer covering the SIS while all electrodes were covered by capsules of biofilm > 1mm and 40% were dislodged or broken. Recording from implanted electrodes were inconsistent and of low quality. Despite poor conditions for the implanted electrodes, the NO ELECTRODE and ELECTRODE groups did not significantly differ in muscle mass, needle CMAP, maximal tetanic force, post fatigue tetanic force, or percent of maximum force recovered (Table 1). However, the NO ELECTRODE group produced significantly more force than the ELECTRODE group during the fatigue protocol starting at contraction number 460 out of 720 (Figure 1).
Conclusions: RPNI muscles were not adversely affected by the presence of an electrode until heavy work was performed by the muscle during fatigue producing excitation. Movement of the muscle against a stiff electrode may have damaged the RPNI muscle. Many electrodes became inoperable due to disengagement and biofouling.
Support: DARPA (N66001-11-C-4190).
Back to 2015 Annual Meeting Program
