Back to 2015 Annual Meeting Program
Outcomes from an Ongoing Multicenter Registry Study on the Use of Processed Nerve Allograft as Compared to Contemporary Controls for Sensory, Mixed, and Motor Nerve Reconstructions
Jason Ko, MD1; Bauback Safa, MD2; Brian Rinker, MD3; Mickey S. Cho, MD4; Dmetry Tuder, MD4; John Ingari, MD5; Emran Sheikh, MD6; Renata V. Weber, MD6; Jozef Zoldos, MD7; Jeffrey A. Greenberg, MD, MS8; Gregory A. Merrell, MD8; Wesley Thayer, MD, PhD9; Gregory Buncke, MD2
1Division of Plastic and Reconstructive Surgery, University of Washington, Harborview Medical Center, Seattle, WA; 2The Buncke Clinic, San Francisco, CA; 3Division of Plastic Surgery, University of Kentucky, Lexington, KY; 4Department of Orthopedics & Rehabilitation, San Antonio Military Medical Center, San Antonio, TX; 5WellSpan Health Orthopedics, York, PA; 6Institute for Nerve, Hand and Reconstructive Surgery, Rutherford, NJ; 7Arizona Center for Hand Surgery, Phoenix, AZ; 8The Indiana Hand to Shoulder Center, Indianapolis, IN; 9Department of Plastic Surgery, Vanderbilt University, Nashville, TN
Introduction: The RANGER registry is an active database designed to collect injury, repair, safety and outcomes data for processed nerve allograft (PNA), Avance® Nerve Graft, AxoGen, Inc. In 2013, a control arm (MATCH) was added to the registry to allow for comparisons of outcomes between conduit and nerve autografts. Here we report our cumulative findings from the ongoing registry on the safety and efficacy of processed nerve allograft with comparisons of outcomes to conduit and nerve autografts.
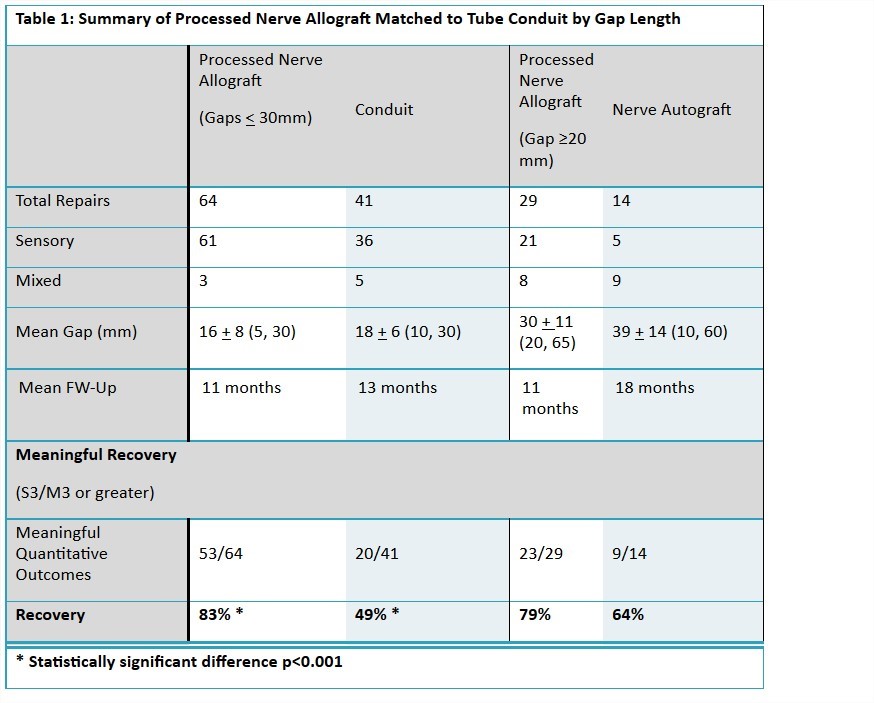
Methods: The RANGER registry is designed to continuously monitor and incorporate data using standardized data collection into a centralized database. For the control arm, a medical record review was conducted at participating centers to identify subjects repaired with conduit or nerve autograft according to the IRB approved protocol. Outcome measures were reported for the cumulative PNA dataset and then stratified for comparisons to controls. PNA repairs with gaps <30mm were compared to conduit and gaps >20mm were compared to the nerve autograft group. Meaningful recovery was defined by the MRCC scale at S3/M3 or greater.
Results: Quantitative outcomes data was available in 109 subjects with 152 PNA repairs. The mean age was 41±16 (18-70). The mean gap was 21±12 (5-65) mm. Recovery of meaningful sensory function was reported in 84% of the repairs (118 sensory/18 mixed). The mean static and moving 2PD was 8±3mm and 7±3mm respectively. Return to light-touch or greater was demonstrated in 47 of 65 repairs reporting SWMF scores. Recovery of meaningful motor function was reported in 68% of repairs (22 mixed/9 motor). There were 8-M3, 6-M4, and 7-M5. No related adverse experiences were reported. PNA was further stratified for comparisons to controls. Meaningful recovery was reported in 49% and 64% in the conduit and nerve allograft groups. See Table 1.
Conclusions: Outcomes from the registry continue to demonstrate the successful use of Avance® Nerve Graft in sensory, motor, and mixed nerve defects between 5 and 65mm. Meaningful recovery at MATCH sites for PNA exceed that of tube conduits and are comparable to nerve autograft. This study is currently in open; additional data incorporated into the registry and MATCH control arms will allow for continued analysis on the role of processed nerve allografts, tube conduit, and nerve autograft in the treatment algorithms for peripheral nerve injuries.
Back to 2015 Annual Meeting Program
